Clarion EDETEK Biometrics Technologies & Services
Simplify , Innovate and Automate Clinical Development Informatics to Accelerate the Delivery of New Treatments
Clarion Analytics, founded by research, the company founders are industry leaders in their relative field of expertise, including telecommunication, Life Science, Bio Structure and Programming, and Bio-Data Management.
Our Products, Solutions and Services
Clarion Analytics and EDETEK through a partnership, offer in Australian market, Clarion-EDETEK end to end Clinical Trials Automation platform capabilities. The product and its associated Clinical Trails Services have been now released to the market.
Both Clarion and EDETEK teams operate as a seamless group in supporting Clinical Trials in Australia catering to small to large clinical trials initiatives. EDETEK platform has been widely used in USA across Biopharma and Clinical Trails Groups across a variety of Clinical Trails. EDETEK provides comprehensive business and technical solutions to advance the science of medicine and improve the quality of life of people around the world.
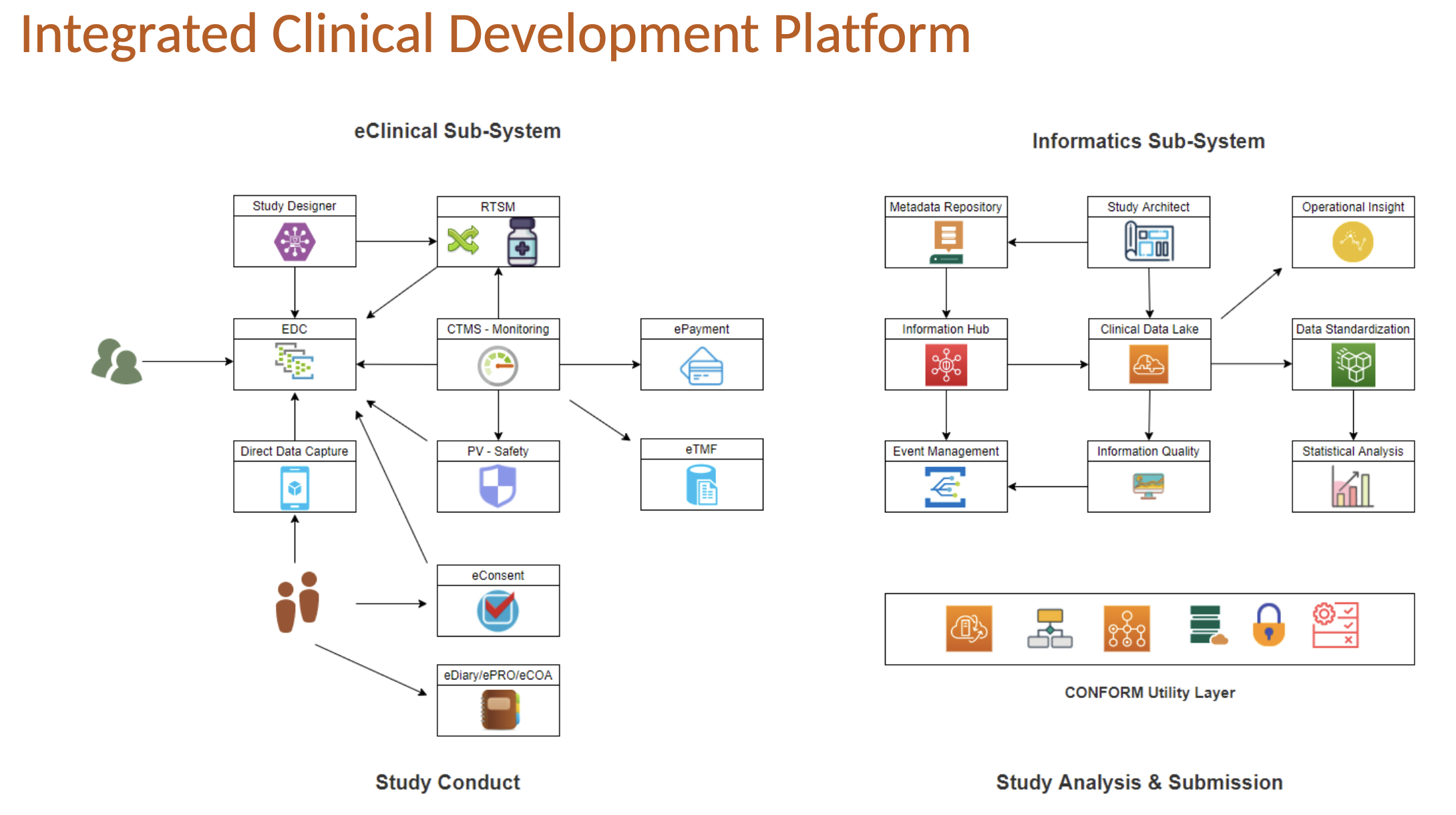
EDETEK’s clinical platforms CONFORM™ eClinical and CONFORM™ Informatics provides data engineering and business analytics needs.Bioinformatics Services are offered from whole of digitization, clinical trials to data analysis and visualization.
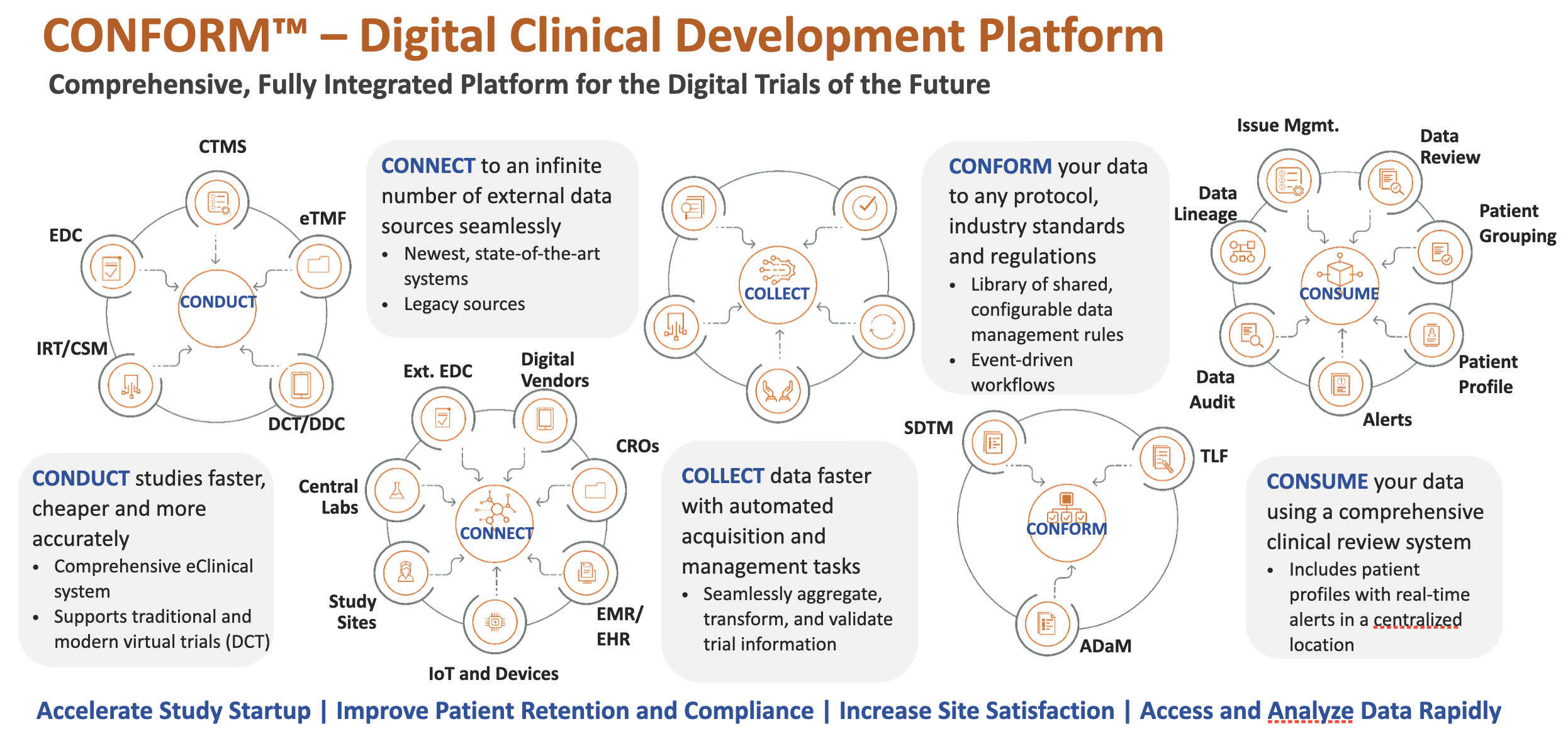
CONFORM™ – Digital Clinical Development Platform
Integrated, end-to-end, turn-key solution that includes EDC, IWRS, Trial Supply Management, CTMS, Safety, eTMF, Payments, eDiary, ePRO/eCOA, Patient Profile, Study Dashboard, etc.
Intuitive user interface, high-performing system
Support complex study designs (repeating cycles, dynamic visits, and special form structures) and randomization schemes (multi-cohorts, multi-paths, multi-stages)
Eliminate redundant data entry/transcription between systems
Lab management module
Web-based CRF design and review, support standard library management
International language support
Central portal access to all studies
Support remote monitoring (source document management and redaction)
Many built-in metrics and reports
Very fast study start-up time
Products
I. CONFORM™ eClinical and all included applications (as licensed and required
by projects) installed and maintained in the AWS Cloud
I. CONFORM™ Informatics and all included applications (as licensed and
required by projects) installed and maintained in the AWS Cloud
Services
i. Clinical Trials Digitization
ii. Project Management
iii. Business Analysis and Analytical services
iv. IT Service Management and Cloud monitoring services
v. Clinical Data Management
vi. Clinical Programming
vii. Biostatistics
For the CONFORM™ Platform (eClinical and Informatics)
i. Configuration
ii. Informatics
iii. Data integration
iv. Application support
v. Training